Altered enzymatic activity of lysozymes bound to variously sulfated chitosans
Chinese Journal Of Polymer Science,
2012,
30,
893-899.
文章链接:http://dx.doi.org/10.1007/s10118-012-1181-8
王宏炜副教授在Chinese Journal Of Polymer Science上发表研究论文
发布日期:2012-08-08
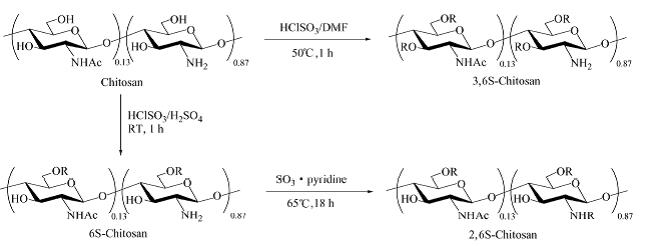
The purpose of this research is to investigate the effects of the variously sulfated chitosans on lysozyme activity and structure. It was shown that the specific enzymatic activity of lysozyme remained almost similar to the native protein
after being bound to 6-O-sulfated chitosan (6S-chitosan) and 3,6-O-sulfated chitosan (3,6S-chitosan), but decreased greatly after being bound to 2-N-6-O-sulfated chitosan (2,6S-chitosan). Meanwhile, among these sulfated chitosans, 2,6S-chitosan induced the greatest conformational change in lysozyme as indicated by the fluorescence spectra. These findings
demonstrated that when sulfated chitosans of different structures bind to lysozyme, lysozyme undergoes conformational
change of different magnitudes, which results in corresponding levels of lysozyme activity. Further study on the interaction of sulfated chitosans with lysozyme by surface plasmon resonance (SPR) suggested that their affinities might be determined by their molecular structures.